
- Core Center:Department of Cell Processing and Transfusion, Research Hospital, The Institute of Medical Science University of Tokyo (IMSUT)
- Principal Investigator:Tokiko Nagamura-Inoue
- FAX:+81-3-5449-5527
- Sub-Core Center 1:Cell Engineering Division, RIKEN BioResource Research Center
- FAX:+81-29-836-9130
概要Overview
 Cord blood (CB) contains an abundance of the most immature hematopoietic stem cells, and is widely used in medical and biological studies, including regenerative medicine, drug discovery, immunology, infection, and genetics research involving iPS cells. The NBRP provides researchers with quality-assured human CB samples that will contribute to the advancement of life science and medical research. The collected CB is processed into several frozen samples at The University of Tokyo’s Cell Resource Center (IMSUT-CRC) and made available to researchers upon request.
Cord blood (CB) contains an abundance of the most immature hematopoietic stem cells, and is widely used in medical and biological studies, including regenerative medicine, drug discovery, immunology, infection, and genetics research involving iPS cells. The NBRP provides researchers with quality-assured human CB samples that will contribute to the advancement of life science and medical research. The collected CB is processed into several frozen samples at The University of Tokyo’s Cell Resource Center (IMSUT-CRC) and made available to researchers upon request.
Stock
・Frozen CB mononuclear cells (Small volume > 1×107/tube)
・Frozen CB mononuclear cells (Large volume > 1×108/bag)
・Frozen CB nucleated cells ( > 3×108/bag)
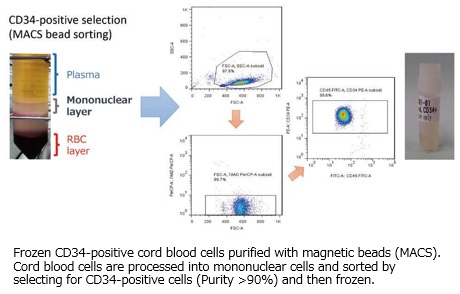
・Frozen CB CD34 positive cells ( > 1×105/tube)

